Diseases can be detected not only directly – by identifying the pathogen (the antigen) – but also indirectly (and retrospectively) by the presence of specific antibodies. The underlying, highly specific antigen-antibody reactions are the basis of serology, a branch of immunology. Serological blood tests are an important element of infection diagnostics and are used to detect disease-specific antibodies in vitro.
The ELISafe 19™ COVID-19 IgG ELISA Detection Kitis designed for the qualitative detection of IgG antibodies against the S1 fragment of SARS-CoV-2 from human serum or plasma samples. This specific detection of the immune response can be used to diagnose or confirm a Covid-19 disease after symptoms have resolved and the infectious phase has ended.
ELISafe 19™ is intended for in vitro diagnostic use only (CE marking for IVD (98/79/EC)) and requires trained medical personnel.
Fields of application
A positive detection with the ELISafe 19™ kit proves that a Covid-19 disease has passed. Accordingly, serological detection of SARS-CoV-2 antibodies plays no significant role in acute diagnostics. IgG antibodies to SARS-CoV-2 are usually detectable no earlier than one week after symptom initiation.
Indications for a serological test with ELISafe 19™ are:
e.g. in patients with mild symptoms or asymptomatic disease. Even if the direct pathogen detection (throat swab) is already negative by means of RT-PCR, the IgG anti-bodies can still be determined over a long period of time.
for clarification of infection chains, evaluation of morbidity and mortality rates and for studies on herd immunity.
Serological diagnostics can provide important information on the social spread of a disease and help to develop effective protective measures and strategies.
of defined groups of people such as medical staff, nurses, etc. to identify recovered and potentially immune individuals.
in critical patients (from 5 days after the first symptoms) and recovered individuals whose plasma may be used for therapeutics.
to support vaccine-related studies.
How long the IgG antibody titer is measurable after SARS-CoV-2 infection (and what the presence or absence of a measurable IgG titer means for the immunity of the individual) is currently unclear, according to the Robert Koch Institute (as of Jan. 14, 2021).
In general, the RKI advises,
„The interpretation of serological test results must be made taking into account the pretest probability, the respective epidemiological situation and knowledge of the specificity/sensitivity values of the test system used.“
How it works
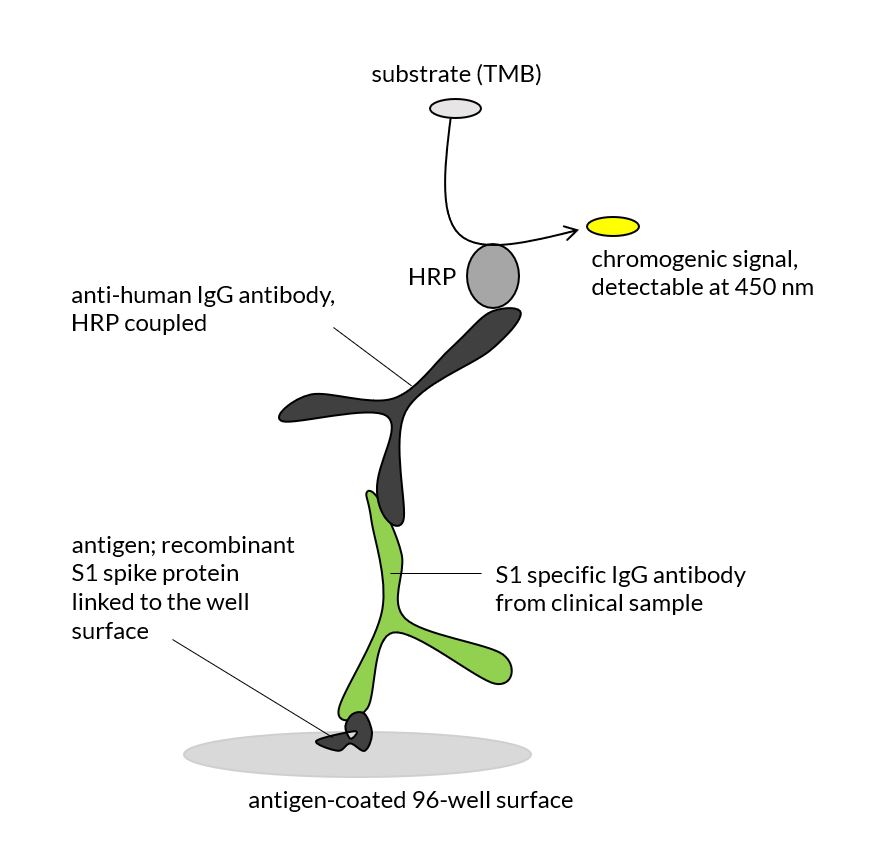
The ELISafe 19™ Kit is designed for the qualitative detection of Covid-19 IgG anti-bodies in human blood. The assay principle is an indirect ELISA test.
The diluted serum or plasma sample of the patient is pipetted into the microwell plate. The surface of each well is coated with the viral antigen (S1 spike protein, recombinant). If Covid-19 antibodies are present in the sample, they bind specifically to the plate surface and can subsequently be detected by HRP-coupled anti-human IgGs. For the detection reaction a chromogenic substrate (TMB) is used, which is read in the ELISA microplate reader (plate reader) at 450 nm.
Kit components
- 96-well microplate coated with the S1 spike protein (including receptor binding site) of SARS-CoV-2
- Plate Sealer
- Various wash, sample and antibody buffers
- HRP-coupled anti-human IgG antibody.
- TMB (3,3′,5,5′-tetramethylbenzidine) and stop solution
- Negative and positive control
Validation
The ELISafe 19™ kit was validated using serum samples from molecularly confirmed (i.e. with positive RT-PCR diagnostics) Covid-19 patients and negative serum samples obtained before the start of the pandemic in January 2020. The calculated sensitivity (when the serum sample was collected 2 weeks after a positive throat swab) was 99%, with a specificity (no false-positive results) of 100%.
